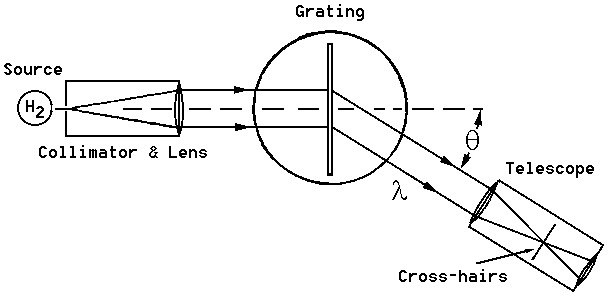
Figure 1: Spectrometer setup.
| PHY 252 | Emission Spectrum of Hydrogen |
In this experiment you will measure the visible part of the hydrogen spectrum, the Balmer series, and determine the Rydberg constant Ry.
If parallel rays of light are incident perpendicularly to the plane of a diffraction grating, with uniform phase over the grating, then one will observe a diffraction pattern which will have a series of intensity maxima at angles q satisfying the equation
n l = d sinq , n = 0, 1, 2,...
where l is the wavelength of the emitted light, and d is spacing between adjacent grooves on the grating. Thus the grating will allow a determination of an unknown wavelength if the positions of the intensity maxima are measured and d is known.
The means for mounting the grating, obtaining parallel rays and measuring the positions of the maxima are provided by the spectrometer table, indicated in the Figure. Light from the source passes through a collimator which consists of a vertical slit and lens. With proper adjustment the collimator can be made to emit parallel rays. This light passes through the grating and is observed in a telescope which has been focused to accept parallel refracted rays. The positions of the various intensity maxima are then determined by noting their position as angles on the vernier scale on the spectrometer table.

Figure 1: Spectrometer setup.
It is crucial for the success of this experiment that the spectrometer is aligned accurately. Your TA will show you how to do this. The steps are the following:
The spectrometer is now ready to measure wavelengths; make sure you understand how to read the vernier scale for the angles. Place hydrogen source so that the light falls into the collimator. Insert the grating in its slot after reading off the spacing d. Now slowly change the angle setting of the telescope until you see a line. Read the angle. Continue until you see the next line (it will have a different colour) and so on. You should see three different colours. The first one is actually most difficult to see. If you rotate still further you will see the same sequence of lines appearing again, but much fainter. This is the second order pattern (n = 2). Try to see as many lines and orders as possible. Repeat this sequence of measurements on the opposite side of the spectrometer. This gives better precision in the measured angles: for each line and order you have now a pair of measured angles qL < 0 and qR > 0, and q = (qR - qL)/2.
An empirical relationship of the form:
| l-1 = a + b/m2 , m = 3, 4, ... | (1) |
can be fit to the hydrogen wavelengths (explain why). Plot your values of 1/l versus 1/m2. Hint: For the last line you see in a given order m = 3 (explain why!). Determine a and b from your graph and, by comparing equation (1) with the Rydberg formula for hydrogen, determine Ry. Include an analysis of errors!