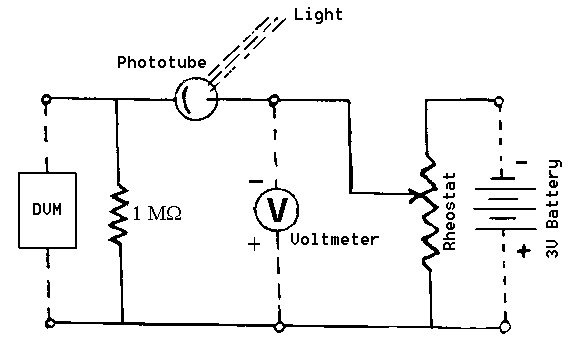
Figure 1: Photocell and connection diagram
| PHY 252 | The Photoelectric Effect |
In this experiment you will use the photoelectric effect to measure the Planck constant h. This classical experiment led to the first precise determination of h, and in 1926 R.A. Millikan received the Nobel Prize for it.
A phototube is illuminated by light of a known wavelength. Electrons are ejected from the photocathode with some kinetic energy K. They are collected as anode current unless a variable retarding potential V is large enough to stop the electrons. For a given potential V all electrons with K < eV will be stopped, and at some value V0 even the fastest electrons with a kinetic energy Kmax will be stopped when
| Kmax = hn - W = e V0 , | (1) |
with n the frequency of the incident light, and W the workfunction of the cathode material. Derive and explain the meaning of this equation. By measuring V0 for different wavelengths (and n) one can determine h/e and, since e is known, h.
The set-up is sketched in the Figure and consists of a phototube, a rheostat to adjust the retarding voltage, a mercury arc light source with different color filters, a battery to supply retarding voltage, measured by a voltmeter, and a 1 MW resistor plus a digital voltmeter (DVM) to read the anode current as a voltage across the resistor.

Figure 1: Photocell and connection diagram