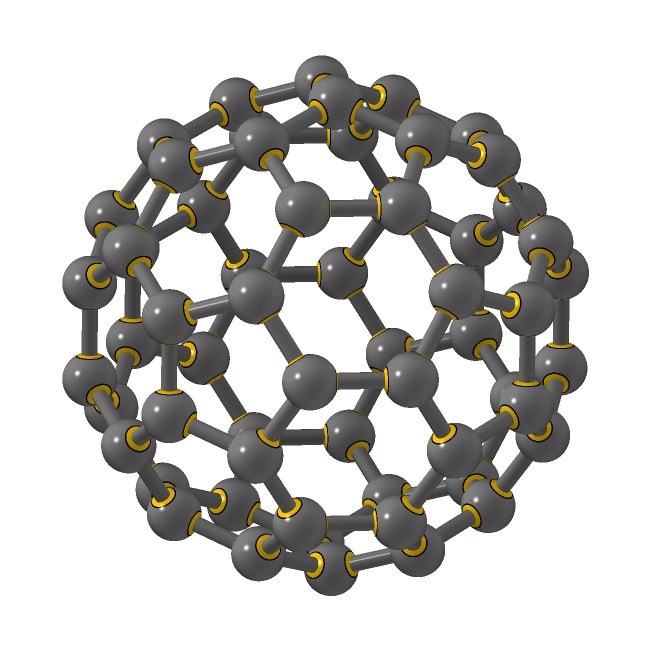
 This robust form of
carbon (C60) remains intact when cooled
into a crystal. Discovered by Kroto and
Smalley. P. W. Stephens and L. Mihaly did
important early research on solid phases. Zeilinger
et al. did quantum interference experiments.
|
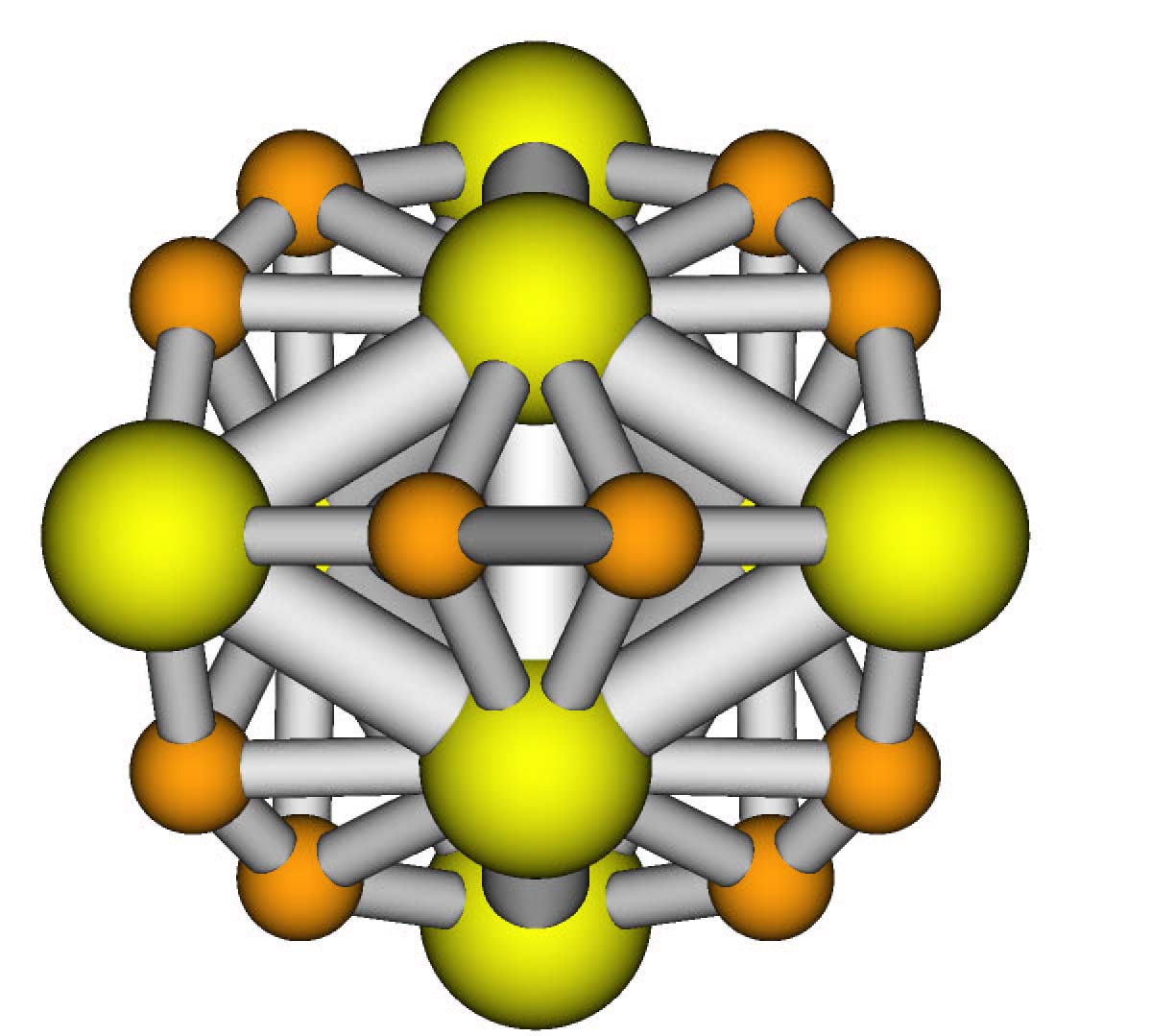
This nice molecule (Mo8C12) only
exists in gas phase. It reacts to make other compounds
in condensed phases. Disovered in molecular beams
by Castleman et al.,
drawn by J. Muckerman |
|
midterm exam Friday Oct. 21 2004 final exam 2007 final exam answers to 2007 final exam Final exam Tuesday Dec. 13, 11:15 am-1:45pm Note: lecture notes will be posted on "Blackboard" |
numbers to memorize equations to memorize shell model and long-range forces phonon notes linear response theory creation operators 2nd quantized notations mean field & Hartree Fock theory 2x2 matrix algebra (BCS) |
|
Homework #1 due Fri. Sept. 9 Homework #2 due Fri. Sept. 16 Homework #3 due Fri. Sept. 23 Homework #4 due Fri. Oct. 7 Homework #5 due Fri. Oct. 14 Homework #6 due Mon. Oct. 31 Homework #7 due Wed. Nov. 9 Homework #8 due Fri. Nov. 18 Homework #9 due Fri. Dec. 9 |
data base; periodic table of the Fermi surfaces. |
|
The
text
is
H.
Ibach
and H Luth, Solids State Physics: An
Introduction to Principles of Materials
Science, 4nd Ed. (Springer
2009). For other recommended books,
see the book
list.
|
pedagogical examples of using character tables |
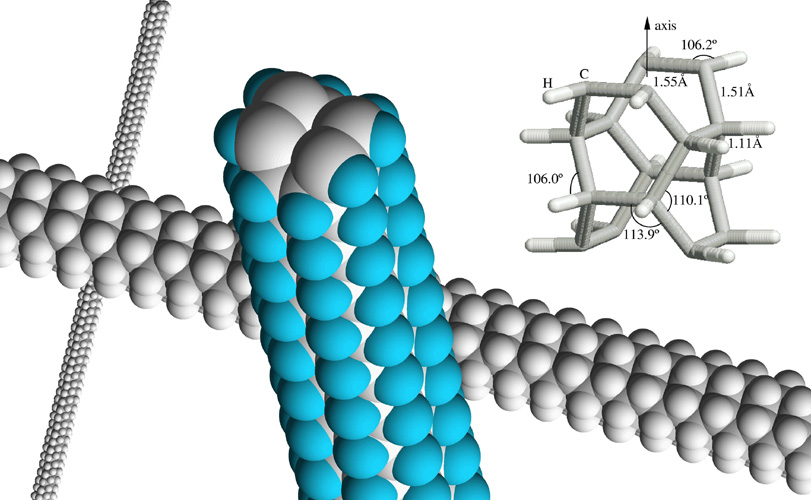 Nanowires, nanorods, and nanotubes are a wonderful area lying nicely between solid state and molecular physics. Chemical reactions catalyzed on the surface of nanoparticles like these are a hot area of research. |
|